Abstract
Background
The immunological effect of human leukocyte antigen (HLA) disparity has not been fully elucidated by the number and locus of HLA antigens or alleles. Several methods to predict epitopes recognized by the immune system have been developed to understand the immunogenicity of amino acid sequences in mismatched HLA pairs. We investigated the association between mismatching of HLA antibody-identified epitopes and hematopoietic stem cell transplantation (HSCT) outcomes.
Patients and Methods
This was a retrospective cohort study with 9,991 patients who underwent their first HSCT for hematologic malignancies from unrelated bone marrow donors in the Transplant Registry Unified Management Program (TRUMP). HLA epitope mismatches (EMM) were quantified using HLA-matchmaker (HLAMM). We conducted a multivariate analysis using Cox proportional hazard regression for the overall survival and the Fine-Gray regression model for competing risks. Statistical analyses were performed with Stata version 15.1 and R version 4.0.2.
Results
The median follow-up period for survivors was 6.3 years (interquartile range [IQR], 2.5-9.2 years). The most common indication for HSCT was acute myeloid leukemia (n=3,917, 39.2%), followed by acute lymphoblastic leukemia (n=1,913, 19.2%), and mature lymphoid malignancies (n=1,906, 19.1%). HSCT from HLA-matched, HLA 1 allele mismatched, and HLA 2 or more allele mismatched donors was received by 6,200, 2660, and 1131 patients, respectively. The number of EMM in recipient-donor pairs in our study population ranged from 0 to 37 in HLA class I (median, 0) and 0 to 60 in HLA class II (median, 1). Patients were categorized into two groups, low and high EMM, using the median value for each epitope matching as the threshold.
Higher HLA class I EMM in the graft-versus-host (GVH) direction was associated with a significantly higher risk of grade III-IV acute GVHD (aGVHD) (hazard ratio [HR] 1.69, 95% confidence interval [CI] 1.21-2.36). Higher EMM for both class I and class II in the host-versus-graft (HVG) direction was associated with a significantly longer time to neutrophil engraftment (HR 0.78, 95% CI 0.65-0.95). In subgroup analysis limited to HLA 1 allele mismatch, class I high EMM group in the GVH direction had a significant association with higher risk for grade II-IV and grade III-IV aGVHD compared with both class I and class II low-EMM group (HR 1.69, 95% CI 1.16-2.47).
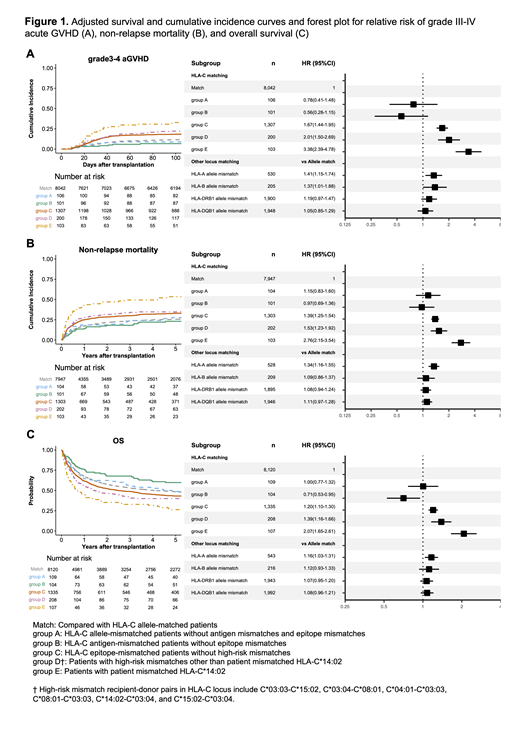
Patients with HLA-C EMM accounted for 94.5% (n=1,603) of patients with HLA class I EMM. We further investigated the impact of HLA-C on severe aGVHD in relation to other known high-risk mismatch patterns. All killer immunoglobulin-like receptor (KIR)-ligand mismatched recipient-donor pairs (n=376) had HLA-C EMM. In multivariate analysis, patients with KIR-ligand mismatches and EMM did not show a higher incidence of grade III-IV aGVHD compared with KIR-ligand-matched patients with EMM (HR 0.96, 95% CI 0.74-1.25). In addition to the known high-risk mismatch patterns in the Japanese cohort (Kawase et al. Blood. 2007, Morishima et al. Haematologica. 2016), EMM was associated with a higher risk for grade III-IV aGVHD (Figure 1A, compared with HLA-C allele-matched patients (Match), HLA-C allele-mismatched patients without antigen mismatches, and EMM (group A): HR 0.78, 95% CI 0.41-1.48; HLA-C antigen-mismatched patients without EMM (group B): HR 0.56, 95% CI 0.28-1.15; HLA-C epitope-mismatched patients without high-risk mismatches (group C): HR 1.67, 95% CI 1.44-1.95; Patients with high-risk mismatches other than patient mismatched HLA-C*14:02 (group D): HR 2.01, 95% CI 1.50-2.69; Patients with patient mismatched HLA-C*14:02 (group E): HR 3.38, 95% CI 2.39-4.78). HLA-C epitope-mismatched patients without high-risk mismatches also showed a higher incidence of non-relapse mortality (NRM) and lower overall survival (OS) than HLA-C allele-matched patients (NRM (Figure 1B): HR 1.39, 95% CI 1.25-1.54; OS (Figure 1C): HR 1.20, 95% CI 1.10-1.30).
Conclusion
HLAMM-based epitope matching might be useful for identifying high-risk groups who can develop serious complications after HSCT from HLA-mismatched unrelated donors. In the HLA-C locus, epitope-mismatched recipient-donor pairs are non-permissive mismatched patterns along with known high-risk amino acid substitutions. Our findings might be helpful for clinicians in selecting permissive donors from alternative donor options.
Iwasaki: Amgen Astellas BioPharma: Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria; Bristol-Myers Squibb Co: Honoraria; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Eisai: Research Funding; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin Co., Ltd.: Honoraria; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; NextGeM Inc: Patents & Royalties; Novartis Pharma K.K.: Honoraria; Ono Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Sanofi K.K.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceutical Company Limited: Honoraria, Membership on an entity's Board of Directors or advisory committees; TEIJIN PHARMA LIMTED.: Honoraria. Uchida: Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Novartis Pharma Inc.: Honoraria. Kataoka: Ono Pharmaceutical: Honoraria, Research Funding; Celgene: Honoraria; Eisai: Honoraria, Research Funding; Astellas Pharma: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; AstraZeneca: Honoraria; Sumitomo Dainippon Pharma: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Janssen Pharmaceutical: Honoraria; Takeda Pharmaceutical: Honoraria, Research Funding; Otsuka Pharmaceutical: Honoraria, Research Funding; Asahi Genomics: Current holder of individual stocks in a privately-held company; Shionogi: Research Funding; Teijin Pharma: Research Funding; Japan Blood Products Organization: Research Funding; Bristol-Myers Squibb: Research Funding; Mochida Pharmaceutical: Research Funding; JCR Pharmaceuticals: Research Funding; MSD: Research Funding. Kanda: MSD: Honoraria; Sanofi: Research Funding; Otsuka Pharmaceutical: Honoraria, Research Funding. Ichinohe: Daiichi Sankyo: Research Funding; Bristol-Myers Squibb: Honoraria; Chugai Pharmaceutical: Research Funding; CSL Behring: Honoraria, Research Funding; Eisai Co.: Honoraria, Research Funding; FUJIFILM Wako Chemicals.: Honoraria, Research Funding; Kyowa Kirin Co.: Honoraria, Research Funding; Ono Pharmaceutical Co.: Honoraria, Research Funding; Nippon Shinyaku Co: Research Funding; Otsuka Pharmaceutical Co.: Research Funding; Sumitomo Dainippon Pharma Co.: Honoraria, Research Funding; Taiho Pharmaceutical Co.: Research Funding; Takara Bio Inc.: Research Funding; Zenyaku Kogyo Co.: Research Funding; Celgene: Honoraria; Novartis Pharma K.K.: Honoraria; Repertoire Genesis Inc.: Honoraria, Research Funding; AbbVie Pharma: Research Funding; Astellas Pharma: Honoraria, Research Funding; Takeda Pharmaceutical Co.: Honoraria. Atsuta: Mochida Pharmaceutical Co., Ltd.: Speakers Bureau; Astellas Pharma Inc.: Speakers Bureau; AbbVie GK: Speakers Bureau; Kyowa Kirin Co., Ltd: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal